Fluorine has an atomic number of 9 and a mass number of 19. The atomic number tell us how many protons and electrons an atom has. To tell how many neutrons we must subtract the atomic number from the mass number. Protons & Electrons = 9. So for the element of FLUORINE, you already know that the atomic number tells you the number of electrons. That means there are 9 electrons in a fluorine atom. Looking at the picture, you can see there are two electrons in shell one and seven in shell two. Similarly, what is the total number of electrons in a fluorine molecule f2?
Electrons are always moving around the nucleus and so possess potential and kinetic energy. But they can only possess certain values of energy, or specific energy levels. (Credit should be given to Niels Bohr for proposing this theory.)
According to Bohr's model of the atom, electrons orbit about the nucleus much like the way planets orbit the sun. Different energy levels are associated with the different orbits. The diagram below shows the Bohr model for fluorine. The nucleus of fluorine has 9 protons. Surrounding the nucleus of fluorine is 9 electrons. The electrons arrange themselves in 3 orbits:
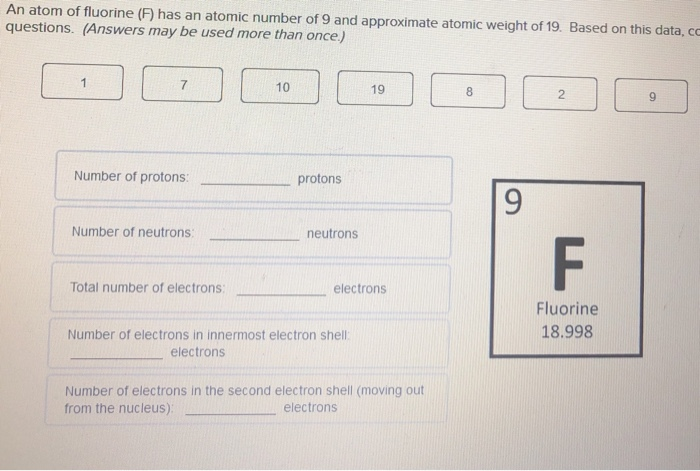
- In the first orbit, there are 2 electrons.
- In the second orbit, there are 7 electrons.
- In the third orbit, there are no electron.
Bohr deduced that:

- electrons inside an atom possess different energies
- electrons in the first orbit belong to the first energy level
- electrons in the second orbit belong to the second energy level
- electrons in the third orbit belong to the third energy level...... etc ......
- each energy level of an atom could only accommodate a certain number of electrons. The maximum number of electrons that can populate a certain energy level is given by the following formula.
- where n = the specific energy level
- For example:
- The maximum number of electron in the first energy level (n = 1) is 2 (1)2 = 2 electrons
- The maximum number of electron in the second energy level (n = 2) is 2 (2)2 = 8 electrons
- The maximum number of electron in the third energy level (n = 3) is 2 (3)2 = 18 electrons
- The maximum number of electron in the fourth energy level (n = 4) is 2 (4)2 = 32 electrons
- The maximum number of electron in the fifth energy level (n = 5) is 2 (5)2 = 50 electrons
- The maximum number of electron in the sixth energy level (n = 6) is 2 (6)2 = 72 electrons
- The maximum number of electron in the seventh energy level (n = 7) is 2 (7)2 = 98 electrons
- Electrons fill the principal energy levels starting from n = 1 to n = 7.
How to draw a Bohr diagram
1. For a hydrogen atom, H, the one electron goes into the first energy level.
|
2. For a helium atom, He, the two electrons go into the first energy level.
|
3. For a lithium atom, Li, two of the three electrons go into the first energy level. The third electron goes into the second energy level. This electron in the outer energy level is called the valence electron. The two electrons in the first energy level are called the core electrons.
Number Of Protons And Electrons For Fluorine
|

Number Of Electrons In Fluorine 19
Bohr diagrams
Content suitability
BCIT courses:CHEM 0011

